Grand Jury Day 3 – PCR Test (English), February 14, 2022, Covid Investigative Committee. This video can be found on Odysee, and the duration is 5:00:57. Actual presentation by Bryan Ardis, occurred on February 13, 2022 starts at the 02:14:54 and ends at 03:19:44
Beginning at the 2:14:15 mark, Bryan Ardis is introduced.
Ardis is identified in the caption after he begins to speak as a “Physician, Holistic Practioner and CEO of ArdisLabs.com, USA”
(Comment: Looking Ardis on the internet he is identified as a chiropractor. So identifying him as a physician is erroneous but since the Model Grand Jury is being conducted by Germans there may simply be a cultural misunderstanding of Ardis credentials within the U.S. medical community. Even though this is a misstep in identifying Ardis’s medical credentials, this still does not necessarily negate the information he brought forward regarding his hypothesis.
I simply wanted to highlight information he brought forward that I thought could be of particular interest. The presentation is in-depth and Ardis speaks rapidly as he has only 20 minutes to give the information that he feels needs to be addressed. I would encourage you to watch the referenced video and in particular the section in which Ardis gives his presentation: 02:14:54 to 03:19:44).
(Comment: As I slowly go through the slides it has dawned on me that the African human population appears to me to being used to test experimental drugs by governments and pharmaceutical corporations.)
Ardis gave a presentation in which he hypothesizes as to what is behind the symptoms found in hospitalized Covid-19 patients. Testimony runs from 02:14:54 to 03:19:44.
(Comment: So what I have done is put down the approximate time markers at which Ardis discusses information that could be of possible interest regarding his hypothesis. Again, I have highlighted some of the major points as the presentation is in-depth. I included the slides that he presented along with the approximate time marker in which it is shown. I have placed arrows to point out what he was talking about, if not obvious in the slides, to make it easier to ascertain. I have designated the slides as 1, 1A, 2 etc so it is easier to follow what is being discussed. I have also linked the full studies that Ardis has cited and/or other info which he refers, to but not included in slide presentation, if I can find it on the internet. And if I happen to stumble upon an article on the internet that could be of possible interest in regards to Remdesivir I will indicate it beginning with Comment:
Also Remdesivir is not capitalized within sentences, but I have taken the liberty to capitalize it, since it is subject matter of Ardis’s hypothesis. I will also note at this time that Remdesivir became known as Veklury at some point, so Remdesivir as of least February 13, 2022 is known as Veklury – – Remdesivir aka Veklury.).
02:13:35: Dr. Reiner Fuellmich, an international trial lawyer, makes an introduction in regards to the presentation that Bryan Ardis will give.
Fuellmich: “Let us now take a closer look at how in reality the world is dealing with this pandemic which is, without any basis. I would like to first focus on those who claim that, will, but that many people are dying, people are dying in Bergamo (Italy), people are dying in New York (U.S.A). So let us start with taking first a look at why that happened, and then we will take a closer look at how else, how can it be treated – I mean we all know there is a virus out there, but how can it be treated? Lets first take a look at the question – Why is this happening and Bryan Ardis is with us. Dr (in chiropractic) Bryan Ardis is with us from Texas (U.S.A.)”
“How do you explain the fact that so many people seemed to have died in New York, for example, of the Corona or from the Corona virus?”
02:14:31 Ardis’s begins his presentation by an introduction that goes from 02:14:29– 02:23:44
02:14:29 – 02:15:21 Ardis discusses symptoms of Corona/Covid-19 symptoms that medical doctors were confronting in hospitals in New York City. Doctors had reportedly noted that they had never seen a respiratory virus that within 3-4-5 days of treatment the respiratory virus had gone from the lungs, and starts attacking the kidneys, causing acute kidney failure. Doctors had never seen it before.
02:15:54 Discusses reading a memo from Anthony Fauci in mid-May 2020 on the NIH site in regards to the treatment of Covid-19 within hospitals and the only drug allowed Remdesivir (later known as Veklury).
(Comment: Tried to find referenced memo on the internet, looked everywhere. The internet mentioned that Fauci released a memo in regards to the FDA granting the Emergency Use Authorization on May 1, 2020, can’t find memo from Fauci in regards to NIH protocol in mid-May 2020. I’m sure it is out there somewhere.)
(Comment: I found an interesting article on the internet published by Market Watch, May 4, 2020, “FDA grants Gilead’s remdesivir emergency authorization for Covid-19 treatment.” Gives you an idea of what was being said about Remdesivir and various responses regarding the use of remdesivir in this time frame of May 4, 2020. See link: https://www.marketwatch.com/story/how-gileads-remdesivir-became-the-leader-in-the-rush-to-find-a-treatment-for-covid-19-2020-05-01)
02:16:52 Discusses his (Ardis) initial investigation as to why Fauci was promoting Remdesivir and the studies supporting the use of Remdesivir.
02:17:53 Ardis discusses the study of Remdesivir used as a treatment for Ebola in which Fauci claimed it had been effective. (Comment: In the treatment of Ebola.)
02:18:00 Ardis could not believe what he had found regarding the studies (Comment: Regarding Remdesivir). What Ardis then learned that Remdesivir was not proven safe and effective for Ebola. Remdesivir was found for Ebola to be the least effective and the deadliest drug in the trial. The safety board for the Ebola trial suspended its use for the rest of the trial. No one was allowed to get it (Remdesivir). Found to have a mortality rate of over 50% out of all of the four drugs in the trial.
02:18:42 At this point Ardis knew that Anthony Fauci was lying about Remdesivir.
02:18:46 Ardis then discusses the second study (Comment: on Remdesivir) which was conducted by Gilead Sciences, Inc. Gave Remdesivir for 10 days to Covid-19 patients – 53 patients in March 2020. Reported conclusions were: 31% of everyone that they gave Remdesivir to between days 5-10 of the treatment – only for 10 days – experienced multiple organ failure, reported acute kidney failure. The reasons doctors in New York City were seeing kidney failure in the hospitals with a respiratory virus is because they had never used Remdesivir before. Ardis notes that doctors had no idea that the drug they were administering was causing acute kidney failure, liver failure, heart failure which are now all published side effects of Remdesivir.
02:19:46 Ardis continues his introduction: “The disappointing part of this, for the entire world, I want you to know, Anthony Fauci said that in May of 2020 that he had asked our federal government here in the United States to buy up all of the stock of this experimental drug, this failed drug, deadly drug and he asked the federal government of the United States to not export the drug to any other country until the end of 2020. This is significant because at the end of 2020 and now in February 2022, America still has the highest death totals of all countries in the entire world for Covid-19 treatment. So all Covid-19 citizens in American, no other country has more deaths than we do. And I attribute it to the hospital protocols initiated early on by Dr. Anthony Fauci.
And if you would let me present it, I’ll show you all the evidence that supports my concern around this drug, because right now, not only does the United States of America is the only country with over 900,000 dead Covid-19 citizens, Brazil is number two, and Brazil has over 630,000, and Brazil since the beginning of March of 2021, is only using Remdesivir in all of its hospitals. The correlation in publicized death side effects and deadly side effects of Remdesivir in my opinion, based on the data I can show you all, is that the hospital protocols which includes Remdesivir and now two years later, is published combined side effects is the number one cause of the death of all Covid-19 hospitalized individuals, worldwide.
And now its come to light also, and everyone needs to know this, from the beginning I’ve heard the term, even from the judge, that this is a plandemic. From the beginning, not only in March and April of 2020 was the city of New York allowed to experiment on all New York patients with a drug called Remdesivir to kill a whole bunch of them, causing acute kidney failure, secondary death by pulmonary edema, is what happens when you cause acute kidney failure. Across the Atlantic Ocean, in the U.K., there is an organization called N.I.C.E along with the U.K. parliament, M.H.R.A, they all were supportive what was called “End of Life” care, and they put in this protocol to take in morphine and midazolam into the nursing homes of individuals all around the U.K. , and in March of 2020, they administered this drug in nursing homes and killed 18,000 people in March of 2020, 25,000 in April 2020, but they called them all Covid-19 deaths.
I believe Remdesivir here in the United States, along with midazolam and morphine protocols called “End of Life” care inside the U.K. that which everyone should refer to Clare Wills Harrison, she is an attorney out of the U.K., has done a great job putting this together. John O’Looney on this testifying group here, he can testify as a funeral director, the amount of deaths that were starting in March and April of 2020, only designated as Covid-19 even when none of these people had Covid-19, in March and April 2020 in the U.K.
I believe there was a huge setup to mass murder a whole bunch of people using drugs and drug protocols that were governed, selected by federal health agencies in our governments, to initiate a huge amount of death and then project on the world that there is a virus killing all of these people. And I said from the beginning of May 2020 that they are going to use these death totals in hospitals and in nursing centers . They are going to call it death by a virus called Covid-19, they are going to take those numbers and they are going to sell the media, and all the societies in America and the U.K. that there is a deadly virus going around the world and you need to sign up for our future vaccine program coming up, which we’ll get into later, in this trial. The vaccines by far was the end agenda that they had to mass murder a lot of people and convince them it was a deadly virus.”
02:23:42 Ardis then starts his presentation/discussion regarding his findings along with slides.
02:24:07 Slide 1: The New England Journal of Medicine, Pub: December 12, 2019: A Randomized Controlled Trial of Ebola Virus Disease Therapeutics. The study was started in November 2018. Ardis discusses. (Comment: Click on link for the study: https://www.nejm.org/doi/full/10.1056/NEJMoa1910993 )
Slide 1: The New England Journal of Medicine “Randomized, Controlled Trial of Ebola Virus Disease Therapeutics“
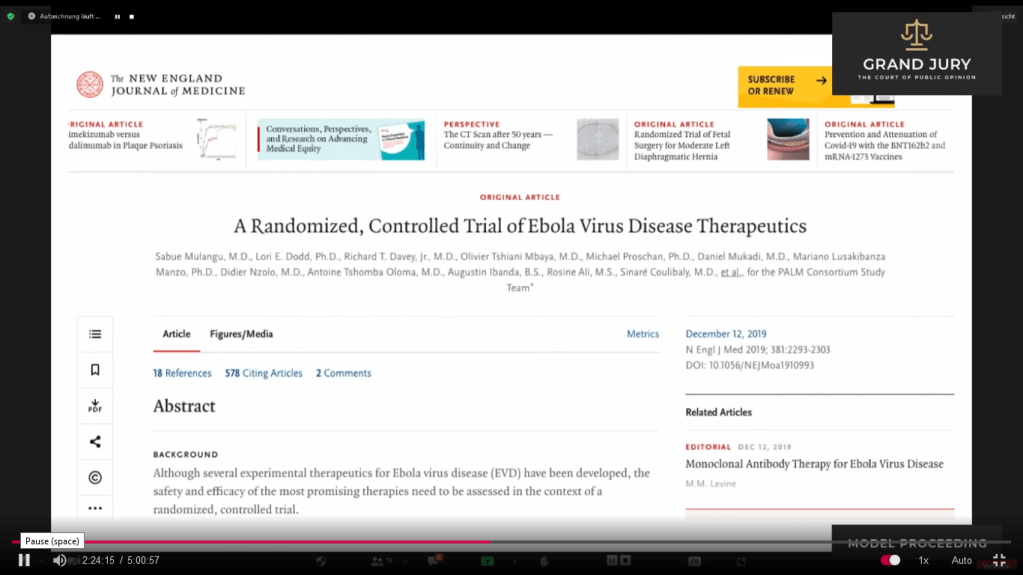
02:24:33 Slide 1A: Methods “All patients received standard care and were randomly assigned in a 1:1:1:1 ratio to intravenous administration of the triple monoclonal antibody ZMapp (the control group), the anti-viral agent remdesivir, the single monoclonal antibody MAb114 or the triple monoclonal antibody REGN-EB3” (Highlighted text). Ardis discusses.
Slide IA: The New England Journal of Medicine “Randomized, Controlled Trial of Ebola Virus Disease Therapeutics ” METHODS
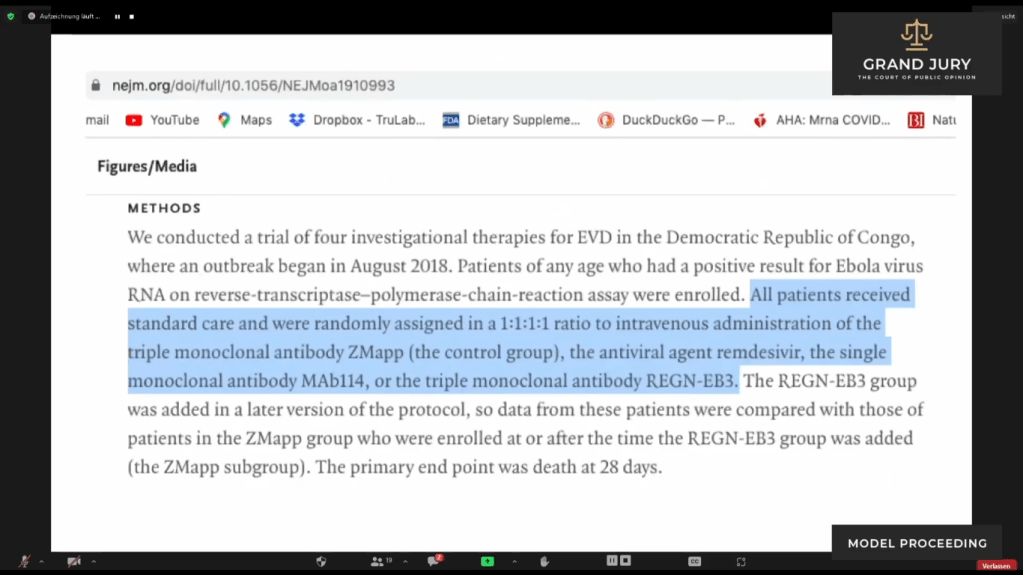
02:24:39 Ardis then discusses how the study was conducted in which four treatments were given – indicated in Slide 1A, highlighted text.
02:25:08 Slide 1B: Conclusion of Ebola Treatment Study – highlighted text. Ardis points out in the conclusion of the study that only three drugs are mentioned: MAb114, REGN-EB3 and ZMapp, when initially in the study there were four drugs
Slide 1B: The New England Journal of Medicine “Randomized, Controlled Trial of Ebola Virus Disease Therapeutics ” CONCLUSIONS

02:26:02 Ardis points out that at the end of the conclusion (shown in parenthesis) (Comment: See arrow) Slide 1B above, that the study was funded by the National Institute of Allergy and Infectious Diseases (NIAID) and others. Ardis then points out the funding by the National Institute of Allergy and Infectious Diseases occurred during November 18, 2018 – December 2019.
02:26:13 Slide Insert 1 Ardis points out that at the time of the aforementioned Ebola treatment study Anthony S. Fauci, M.D. was the director of the National Institute of Allergy and Infectious Diseases (NIAID). Fauci was appointed director of NIAID in 1984.
(Comment: Link to page shown in Insert 1. Click on link for page: https://www.niaid.nih.gov/about/director)
Slide Insert 1: National Institute of Allergy and Infectious Diseases “Anthony S. Fauci, M.D., NIAID Directors“

02:26:41 – 02:27:06 Slide 1C Ardis discusses. The PCR test was used to screen for Ebola . In the highlighted text it indicated that patients were being screened thru the RT-PCR test which included pregnant women. If the pregnant woman were tested positive for Ebola, she were eligible to be included in experimental drug trial in which Remdesivir was one of the drugs being studied. Neonates who were 7 days of age or younger were also eligible if the mother had documented EVD.
Slide 1C: The New England Journal of Medicine “Randomized, Controlled Trial of Ebola Virus Disease Therapeutics ” SCREENING AND RADOMIZATION

02:27:17 Slide 1D Mortality (Highlighted text) Ardis discusses that the safety board (in regards to the aforementioned Ebola treatment study) recommended terminating the random assignment to ZMapp and Remdesivir on August 9, 2019, after 681 patients had been enrolled in the the study and the data and safety monitoring board had conducted interim analysis of 499 patients.
Slide 1D The New England Journal of Medicine “Randomized, Controlled Trial of Ebola Virus Disease Therapeutics ” MORTALITY

02:27:56 Slide 1E The New England Journal of Medicine “Randomized, Controlled Trial of Ebola Virus Disease Therapeutics ” Table 2: Comparison of Death at 28 days According to Treatment Group.
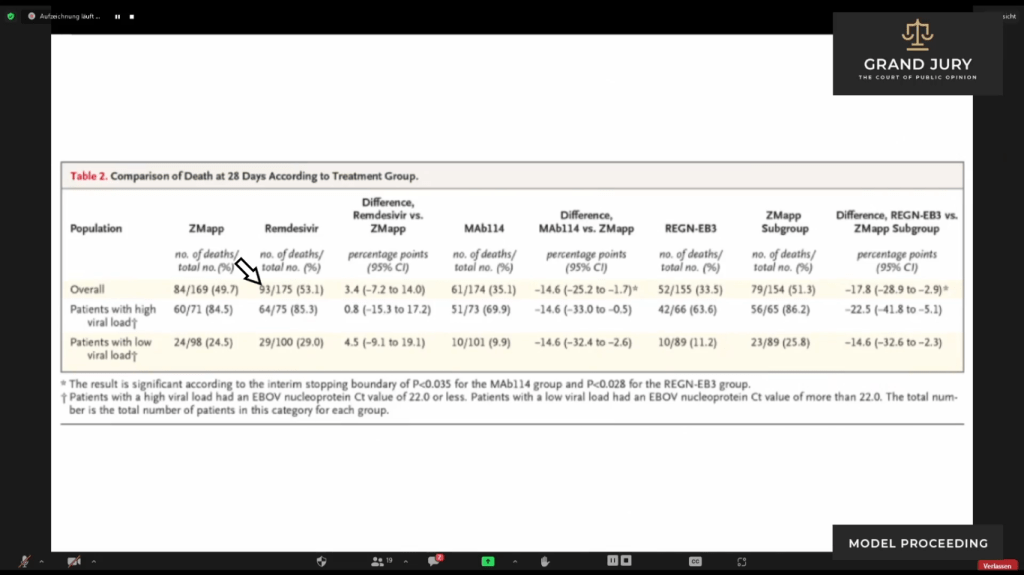
02:28:10 Ardis describes how the aforementioned Table 2 is set up with the drugs listed in the column headings, ZMap, Remdesivir, MAb114, REGN-EB3. Ardis then discusses further the findings regarding each drug pointing out that Remdesivir had a mortality rate of 53.1%.
(Comment: In the first row designated “Overall” is the mortality rate of each drug used in the study. Remdesivir (Comment: See arrow) had a death rate of 93 individuals out of a total of 175 individuals who were given this drug and a mortality rate of 53.1%. Another words out of 175 individuals 93 died so only 82 individuals lived.)
02:30:11 Slide 1F: Author Affiliations.
(Comment: This is in regards to the Ebola study that was published in the New England Journal of Medicine see Slide 1 and their findings: Slides 1A, 1B, 1D, 1E, 1F)
PALM Writing Group second paragraph: Ardis highlights the National Institute of Allergy and Infectious Diseases among others.
(Comment: I also noticed Gilead is listed which might be of interest but is not highlighted – second paragraph under PALM Writing Group – see arrow).
Slide 1F: The New England Journal of Medicine “Randomized, Controlled Trial of Ebola Virus Disease Therapeutics ” Author Affiliations

02:30:21 Slide 2: Compassionate Use of Remdesivir for Patients with Severe Covid-19. Gilead Sciences study
Ardis realized that Remdesivir was not safe and effective for the treatment of Ebola which Fauci had claimed it had been. (Comment: Probably referring to the aforementioned memo at the 02:15:54 time stamp.)
(Comment: Also I happen to find an article published on April 29, 2020 by Daily Mail titled: “FDA may approve emergency use authorization of Ebola drug remdesivir TODAY after Dr Fauci praised the results of one study as ‘proof that a drug can block this virus‘” Notice in the title that Remdesivir is identified as “Ebola drug remdesivir” Say what???? Click on link for article: https://www.dailymail.co.uk/health/article-8270643/FDA-approve-emergency-use-authorization-Ebola-drug-remdesivir-TODAY.html)
Slide 2: NEJM.org “Compassionate Use of Remdesivir for Patients with Severe Covid-19.” Study funded by Gilead Sciences
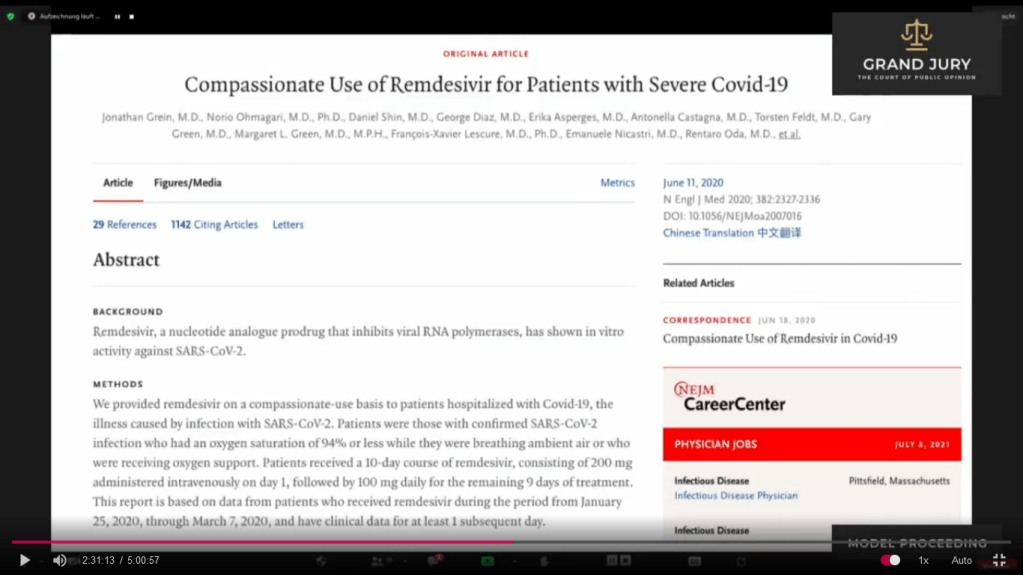
(Comment: So I looked up the document shown on Slide 2 on the internet. For full published study click on link: https://www.nejm.org/doi/full/10.1056/NEJMoa2007016 . It is noted on the site that this study was originally published on April 10, 2020 at NEJM.org . NEJM stands for New England Journal of Medicine. It states at the conclusion that the study was funded by Gilead Sciences. Remember on Slide 1F, I have an arrow showing Gilead as one of the authors of the Ebola study using Remdesivir).
02:30:31 – 02:30:55 Ardis discusses, the aforementioned Gilead study “Compassionate Use of Remdesivir for Patients with Severe Covid-19” was initiated in January 2020 using Remdesivir on COVID-19 patients and was completed in March 2020. Ardis notes that the Ebola study using Remdesivir was published in December 2019 in which Remsdesivir had been pulled from the trial study, yet was now being used in a trial study on COVID-19 patients one month later, January 2020.
(Comment: See last sentence of Slide 2 under Methods: “This report is based on data from patients who received remdesivir during the period from January 25, 2020, through March 7, 2020 and have clinical data for at least 1 subsequent day.”)
(Comment: So this begs the question, if Remdesivir was so lethal for human beings in Africa that they discontinued it as part of the Ebola trial study, how is it now all at once not lethal to give to Covid-19 patients – one month after the findings in Africa was published? Again slides 1, 1A, 1B, 1D, 1E, 1F. I think that this is an honest and fair question to ask.)
02:30:55 Ardis than explains how Gilead had conducted the study using Remdesivir on Covid-19 patients. The study was published June 11, 2020.
02:31:21 Slide 2A The Gilead study using Remdesivir for Covid-19 patients. Ardis discusses findings (highlighted text) in which serious adverse affects were noted: Multiple-Organ-Dysfunction Syndrome; Septic shock; Acute kidney injury and Hypotension.
Slide 2A: NEJM.org “Compassionate Use of Remdesivir for Patients with Severe Covid-19.” Study funded by Gilead Sciences. SAFETY

02:32:18 –02:32:36 Slide 3 Open Access, Pub June 30, 2020; Case report: September 01, 2020 : Case report study of the first five Covid-19 patients treated with Remdesivir in France.
According to Ardis that at the time the Gilead study was taking place another study in France was being conducted. France gave Remdesivir only to 5 people.
Slide 3: Open Access, Pub June 30, 2020; Case report: September 01, 2020 : “Case report study of the first five Covid-19 patients treated with Remdesivir in France”.

(Comment: Click on link to article mentioned in Slide 3: https://www.sciencedirect.com/science/article/pii/S1201971220305282 however just a different publication evidently.)
02:32:48 Slide 3A Ardis discusses what France found in their case study of giving Remsdesivir to Covid-19 patients – highlighted text. Study conducted from January 24, 2020 – March 1, 2020.
(Comment: Remember, Slide 2 Gilead was conducting a study on Remdesivir as a treatment for Covid-19 from January 25, 2020 – March 7, 2020.)
Slide 3A Open Access, Pub June 30, 2020; Case report: September 01, 2020 : “ Case report study of the first five Covid-19 patients treated with Remdesivir in France” Case presentations

2:33:00 Slide 3B Abstract: What the French study found – highlighted text. Ardis then goes over what is being indicated in the highlighted text.
Of the five people in the study, four of the patients in the study treatment were interrupted due to the following: Two of the patients had increased liver toxicity in the blood work – 3 to 5 times the normal range (Comment: This is important to keep in mind for latter information that will be revealed). Two other patients treatments were interrupted due to renal failure, requiring renal replacement (Comment: Another words kidney failure, with kidney replacement aka transplants). So 4 of the 5 patients were pulled from the study due to severe reaction to Remdesivir.
Two out of the five patients died – (Comment: See arrow.
Slide 3B: Open Access, Pub June 30, 2020; Case report: September 01, 2020 : “Case report study of the first five Covid-19 patients treated with Remdesivir in France”. Abstract

02:34:11 Slide 4 Ardis discusses. NBC News reports on November 19, 2020 Remdesivir Shouldn’t Be Used on Hospitalized Covid-19 Patients, WHO Advises. (Comment: Highlighted text)
(Comment: WHO is the World Health Organization. For complete article click on link: https://www.nbcnews.com/health/health-news/remdesivir-shouldn-t-be-used-hospitalized-covid-19-patients-who-n1248320)
Slide 4: NBC News “Remdesivir Shouldn’t Be Used on Hospitalized Covid-19 Patients, WHO Advises“
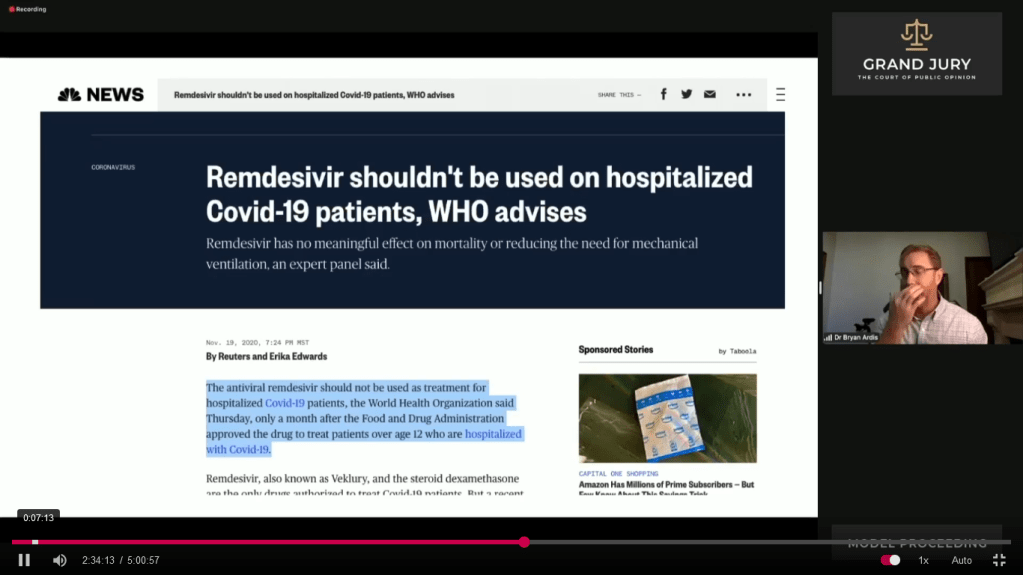
2:34:56 Slide 4A Ardis discusses. Highlighted text. Remdesivir has potential side-effects on the kidneys according to data Gilead shared with the European Medicines Agency.
Slide 4A NBC News “Remdesivir Shouldn’t Be Used on Hospitalized Covid-19 Patients, WHO Advises“

02:35:07 Slide 5 Nature Medicine, Pub July 10, 2020: “Extrapulmonary manifestations of Covid-19“. (Comment: Full article click on link: https://www.nature.com/articles/s41591-020-0968-3)
Highlighted text under Abstract shows symptoms seen in Covid-19 patients other than respiratory. Ardis discusses information highlighted.
Slide 5: Nature Medicine, Pub July 10, 2020: “Extrapulmonary manifestations of Covid-19“.

02:37:09 -02:38:12 Slide 5A Nature Medicine, Pub July 10, 2020: “Renal manifestation: Epidemiology and clincal presentation. Highlighted text. Ardis discusses highlighted section.

02:38:14 – 02:39:20 Slide 5B Ethics declaration Highlighted text A list of those individuals who are contributing to the study. Ardis discusses that a person working for Gilead is listed.
Slide 5B: Nature Medicine, Pub July 10, 2020: “Extrapulmonary manifestations of Covid-19“. Ethics declaration

02:37:17 – 2:39:25 Ardis will point out his hypothesis that the symptoms other than the respiratory symptoms of Covid-19 are the result of the side effects of Remdesivir poisoning.
02:39:28 Slide 6 National Library of Medicine , Epub: January 16, 2021; Clin Pharmaceutical Ther 2021 Apr; “Remdesivir and Acute Renal Failure: A Potential Safety Signal From Dispropportionality Analysis of the WHO Safety Database”. (Comment: For full article click on link: https://pubmed.ncbi.nlm.nih.gov/33340409/ )
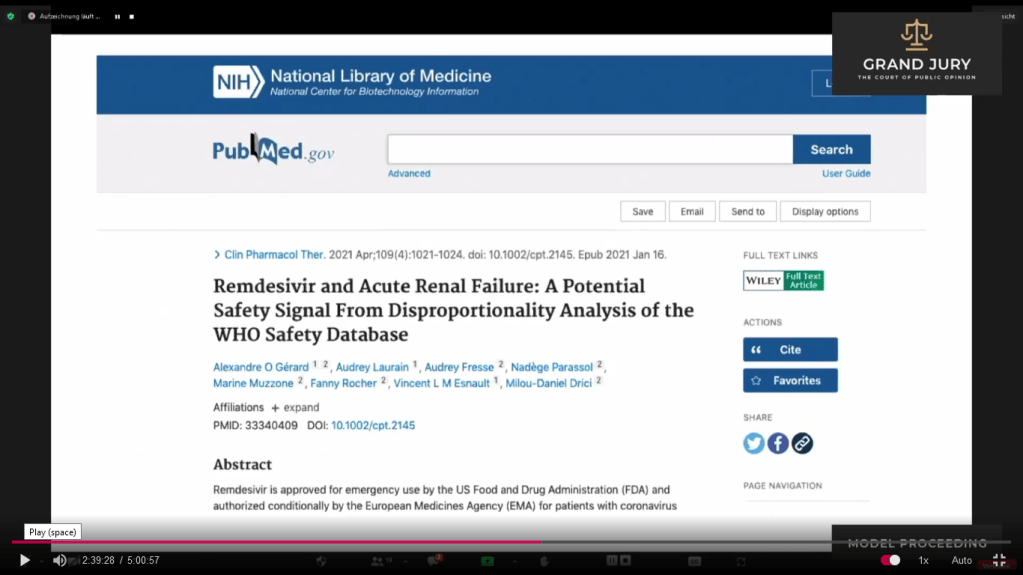
02:39:38 – 02:40:58 Slide 6A Abstract Highlighted. Ardis discusses.(Comment: Basically the WHO database was used to study 4 drugs being used to treat Covid-19 in which the study was trying to determine if Covid-19 is causing acute kidney failure throughout the world. )
Slide 6A: National Library of Medicine , Pub: Date Needed: “Remdesivir and Acute Renal Failure: A Potential Safety Signal From Dispropportionality Analysis of the WHO Safety Database.“

02:40:58 – 02:41:16 Slide 6B What they found. Highlighted text. Ardis indicates first sentence only in discussion.
(Comment: At the bottom of the abstract is shows: ⓒ 2020 The Authors. Clinical Pharmacology & Therapeutics. ⓒ 2020 American Society for Clinical Pharmacology & Therapeutics. See arrow on Slide 6B)
Slide 6B National Library of Medicine , Pub: Date Needed: “Remdesivir and Acute Renal Failure: A Potential Safety Signal From Dispropportionality Analysis of the WHO Safety Database”. Abstract

02:41:16 – 02:42:30 Slide 7 October 13, 2021 Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A Literature Review. Highlighted text. (Comment: For abstract click on link: https://pubmed.ncbi.nlm.nih.gov/34643857/)
Ardis discusses findings.
Slide 7: October 13, 2021 “Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A Literature Review.” Published: pubmed.ncbi.nlm.nih.gov. Highlighted text.

02:42:31 – 02:42:43 Slide 8 May 30, 2020 Chloroquine and Hydroxychloroquine Increase Risk of Death in Covid-19. (Comment: For full text click on link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7256923/)
Basically Ardis discusses that Remdesivir’s side effects are considerably worse than that of Chloroquine in regards to cardiotoxic effects.
Slide 8: May 30, 2020 “Chloroquine and Hydroxychloroquine Increase Risk of Death in Covid-19.” Published on ncbi.nlm.nih.gov.
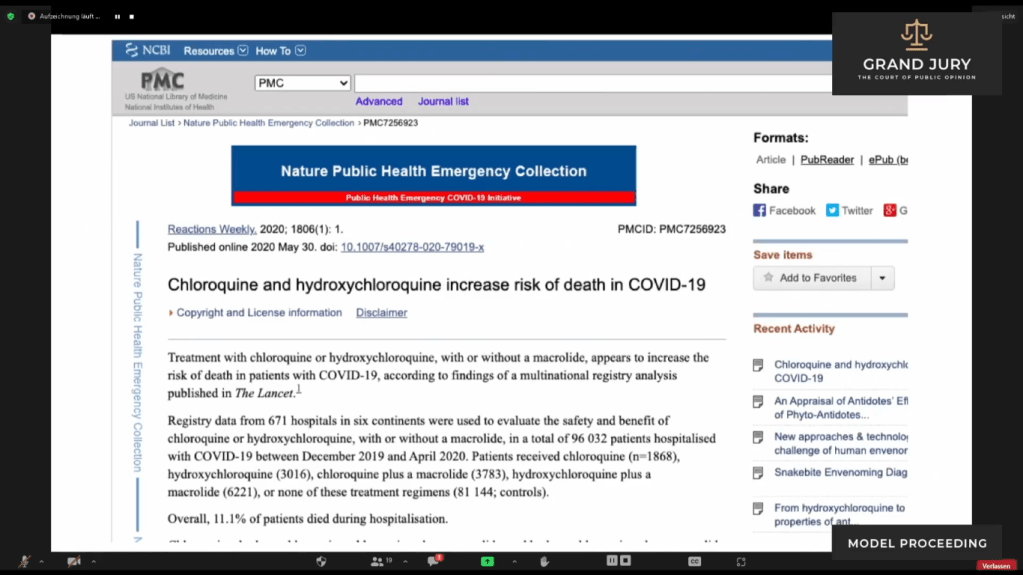
02:42:43–02:42:42 Slide 8A See highlighted text
Slide 8A: May 30, 2020 “Chloroquine and Hydroxychloroquine Increase Risk of Death in Covid-19.” Published on ncbi.nlm.nih.gov.

02:43:04 -02:43:16 – Ardis then points out studies later on found Remdesivir was much worse in regard to affecting the heart than other drugs being used for Covid-19 treatment. Ardis refers back to Slide 7 “Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A literature review” – under Abstract. Then he refers back to this same Abstract with another part of it highlighted which I have named 7.1 (Comment: Both slides listed below for convenience but numerically out of sequence).
Slide 7 “Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A literature review” Abstract

02:43:17 – 02:43:48 Slide 7.1 – “Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A literature review” Abstract
Another section highlighted and discussed by Ardis.

02:43:48 – 02:44:03 Slide 9 Virology Journal Pub August 22, 2005: Chloroquine is a Potent Inhibitor of SARS Coronavirus Infection and Spread. (Comment: For full article click on link: https://virologyj.biomedcentral.com/articles/10.1186/1743-422X-2-69)
Ardis discusses the significance of what is being indicated in the abstract as well as the date of the abstract.
Slide 9 – Virology Journal Pub August 22, 2005: “Chloroquine is a Potent Inhibitor of SARS Coronavirus Infection and Spread“.

02:44:03 – 02:44:22 Slide 9A: Conclusion of study found in Slide 9 – highlighted text. Ardis discusses.
Slide 9A – Virology Journal Pub August 22, 2005: “Chloroquine is a Potent Inhibitor of SARS Coronavirus Infection and Spread“. Conclusion

02:44:22 -02:44:57 Slide 10 NIH Covid-19 Treatment Guidelines: Table 2e: Characteristics of Antiviral Agents That are Approved or Under Evaluation for Treatment of Covid-19. Up Dated July 08, 2021 (Comment: As shown in slide).
(Comment: The actual document Table 2e does not appear to be readily available on the internet. For full document see link to Ardis’s website https://thedrardisshow.com/covid-approved-treatments-and-cms-gov-payouts-for-remdesivir)
02:44:42 Ardis discusses the aforementioned table pointing out the information on the 5th bullet regarding the adverse effects of Remdesivir (Comment: See arrow).
Slide 10 – Table 2e Characteristics of Antiviral Agents That are Approved or Under Evaluation for Treatment of Covid-19. Dated: July 08, 2021

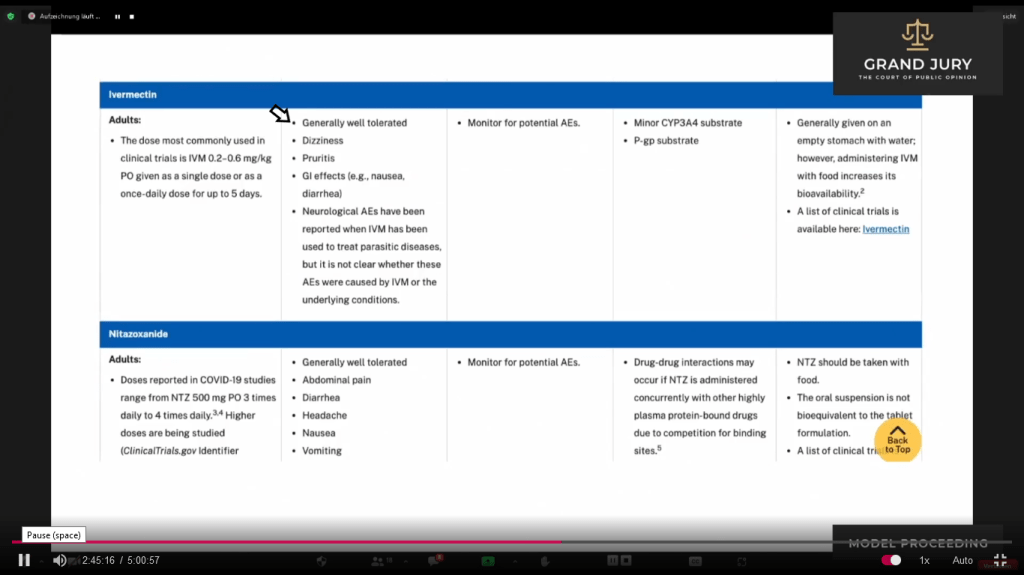
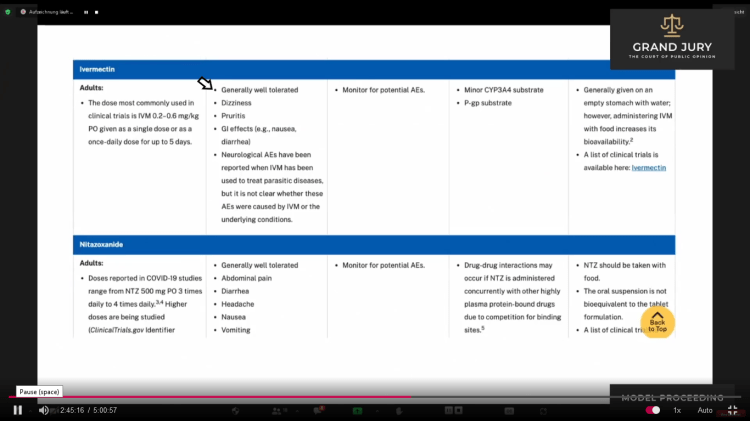
02:44:57 – 02:45:26 Ardis points out the first bullet point (Comment: See arrow) on the adverse side effects of Ivermectin. As shown “Generally well tolerated.” (Comment: This will be important to remember).
Slide 10A – Table 2e Characteristics of Antiviral Agents That are Approved or Under Evaluation for Treatment of Covid-19. Dated: July 08, 2021
02:45:32 – 02:45:46 Ardis then points out that Table 2e has been updated in December 2021 (Comment: December 16, 2021) and is now called “Table 2f Characteristics of Antiviral Agents.” (Comment: See Slide 11)
(Comment: French only used five patients in their study using Remdesivir for the treatment of Covid-19 patients and four were taken off of Remdesivir early because of adverse reaction, evidently 2 died.
Remdesivir was axed early from the Ebola study due to the fact that Remdesivir had a mortality rate 53.1%, with 93 individuals, out of 175 individuals who were given this drug died, and only 82 individuals surviving. May I point out each and everyone of the individuals that died were humanbeings not guinea pigs.
Yet it appears that Remdesivir is the only drug that is being approved for use as treatment for Covid-19 patients in hospitals as of December 2021 — just 3 months ago.)
02:45:46 Slide 11 – NIH COVID-19 Treatment Guidelines Table 2f. “Characteristics of Antiviral Agents”. Last Updated December 16, 2021 (Comment: This table has now been updated as of February 24, 2022 see attached link: https://www.covid19treatmentguidelines.nih.gov/tables/antiviral-characteristics/
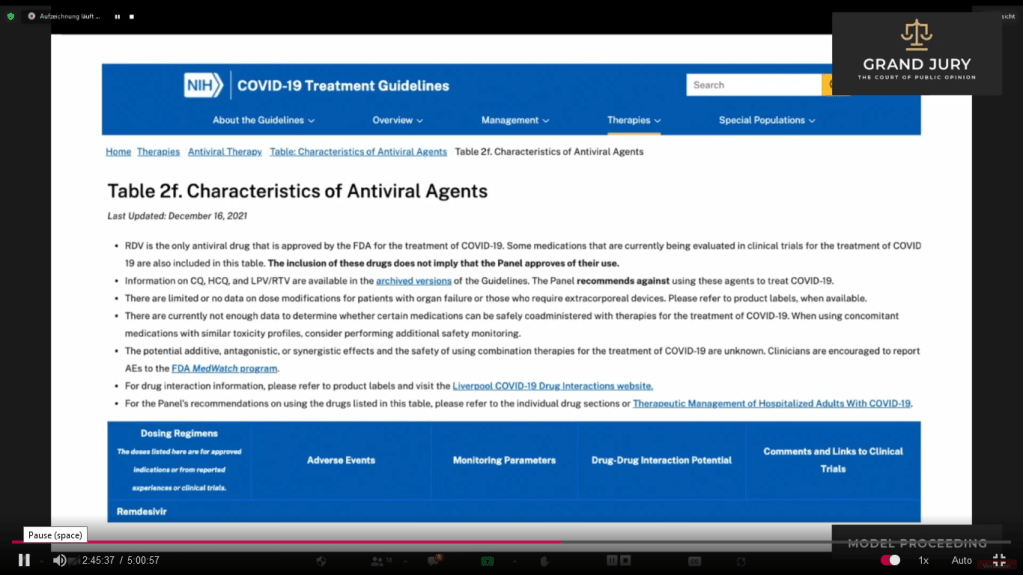
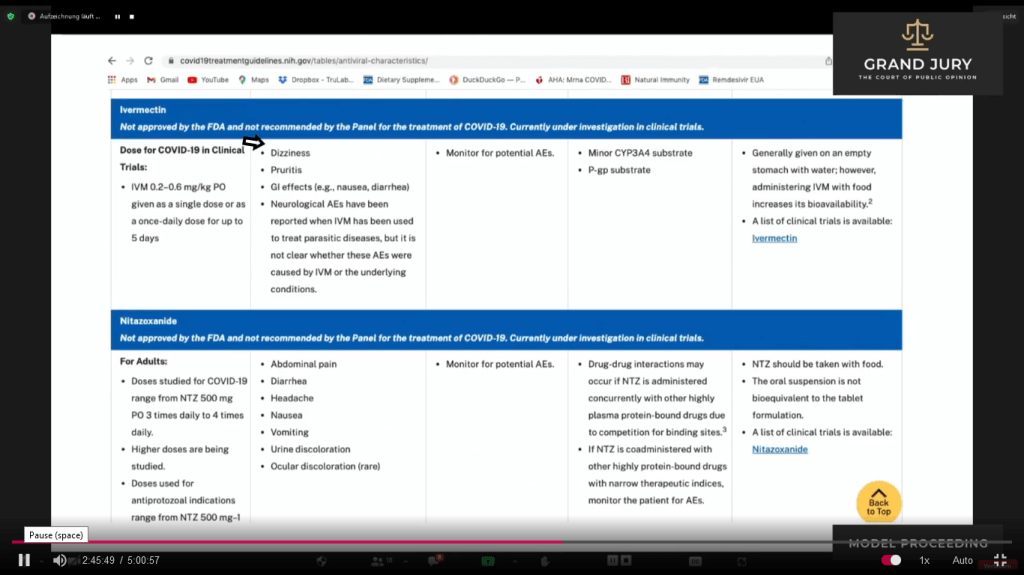
02:45:47 – 02:46:20 Ardis noted that under Ivermectin on Table 2f, that “they” deleted the “Generally Well Tolerated” under the Adverse Events column. (Comment: See Slide 11A, arrow shows that “Generally Well Tolerated” is missing in Table 2f. See below Slide 10A showing the previous Table 2e with “Generally Well Tolerated” – see arrow)
Slide 11A Table 2f “Characteristics of Antiviral Agents”. Last Updated December 16, 2021
Slide 10A NIH Covid-19 Treatment Guidelines: Table 2e: Characteristics of Antiviral Agents That are Approved or Under Evaluation for Treatment of Covid-19. Last Updated July 08, 2021
02:45:54 – 02:46:19 Ardis notes he has been pointing this out to legislators around the country and world.
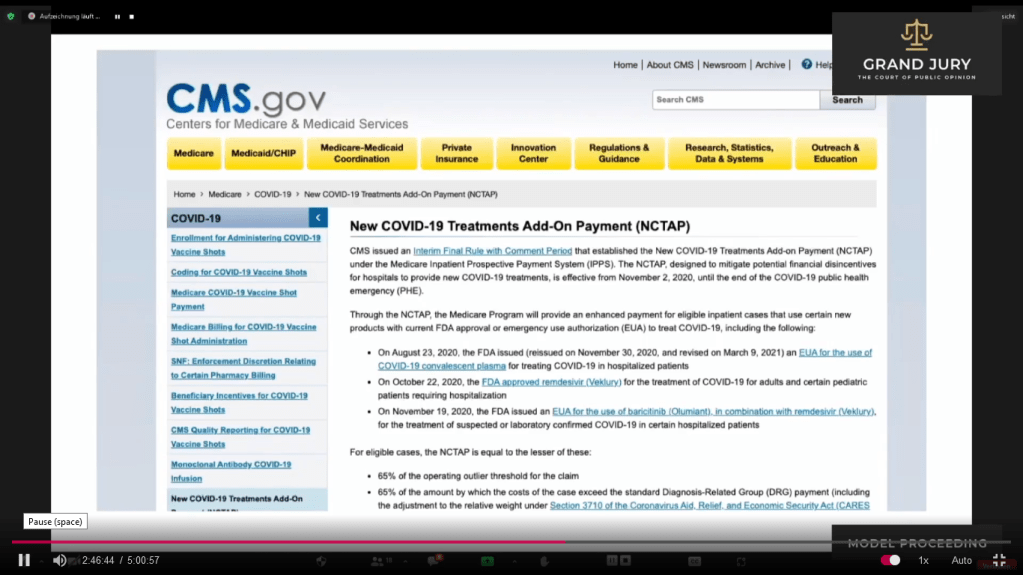
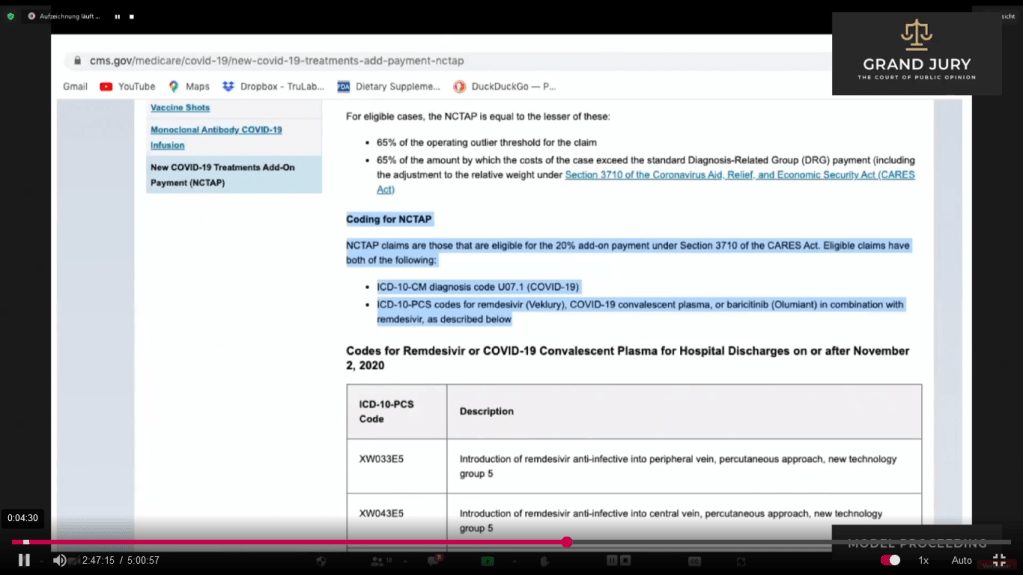
02:46:43 Slide 12 CMS.gov Ardis discusses how Medicare appears to be bribing hospitals to use Remdesivir.
(Comment: CMS stands for Center for Medicare/Medicaid Services. Clink on link for full document: https://www.cms.gov/medicare/covid-19/new-covid-19-treatments-add-payment-nctap ).
Slide 12 CMS.gov “New COVID-19 Treatments Add-On Payments (NCTAP)“
02:47:13 – 02:48:10 Slide 12A CMS.gov “New COVID-19 Treatments Add-On Payments (NCTAP)” Highlighted text Ardis points out that it shows coding for NCTAP in regards to Covid-19 treatment with additional payments being made.
Slide 12A CMS.gov “New COVID-19 Treatments Add-On Payments (NCTAP)” Highlighted text.
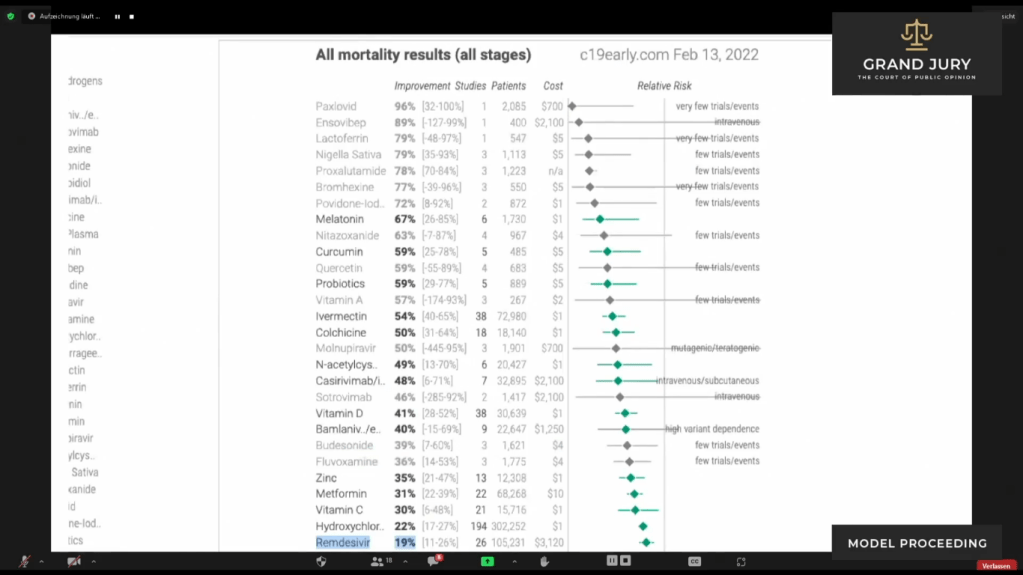
02:48:11 – 02:49:52 Slide 13 CHART “All Mortality Results (All stages)” February 13, 2022.
Ardis points out chart showing treatments being used for Covid-19 patients around the world and the mortality rate for each type of treatment. Ardis asks: ” Why is Medicare continuing to bribe the hospitals?” Ardis discusses that Remsdesivir is still only showing 19% improvement for Covid-19 patients. Ardis then discusses the comparison of improvements as well as costs for the other drugs shown. Ardis questions why Remdesivir is still being used when there are 30 plus other products shown with better results two years later after the initial use of Remdesivir.
Slide 13 CHART “All Mortality Results (All stages)” February 13, 2022. ( See highlighted text for Remdesivir.)

02:49:53-02:50:20 Slide 14 FDA “Coronavirus (Covid-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat Covid-19 Due to Omicron Variant” January 24, 2022. Ardis discusses.

02:50:20 – 02:50:25 Slide 14A FDA “Coronavirus (Covid-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat Covid-19 Due to Omicron Variant” January 24, 2022 (Highlighted text) Ardis discusses.

02:50:25 – 02:50:47 Slide 14B FDA “Coronavirus (Covid-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat Covid-19 Due to Omicron Variant” January 24, 2022 (Highlighted text) Ardis discusses.

02:50:48 – 02:51:42 Ardis discusses drugs accepted for use for the treatment of Covid-19 (Remdesivir) in regards to the aforementioned information in Slide 14B in the U.S. and again compares it to the information on the “All mortality results” chart
Slide 14C CHART “All Mortality Results (All stages)” February 13, 2022. ( See highlighted text for Remdesivir.)
02:51:43 Slide 15 FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….” (Comment: See Slide 15 for the complete title of this fact sheet as it is rather long).
(Comment: Veklury previously known as Remdesivir. Its my personal opinion that they have conveniently changed the name of Remdesivir to Veklury to further confuse and muddy the medical waters).
Slide 15 FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….”
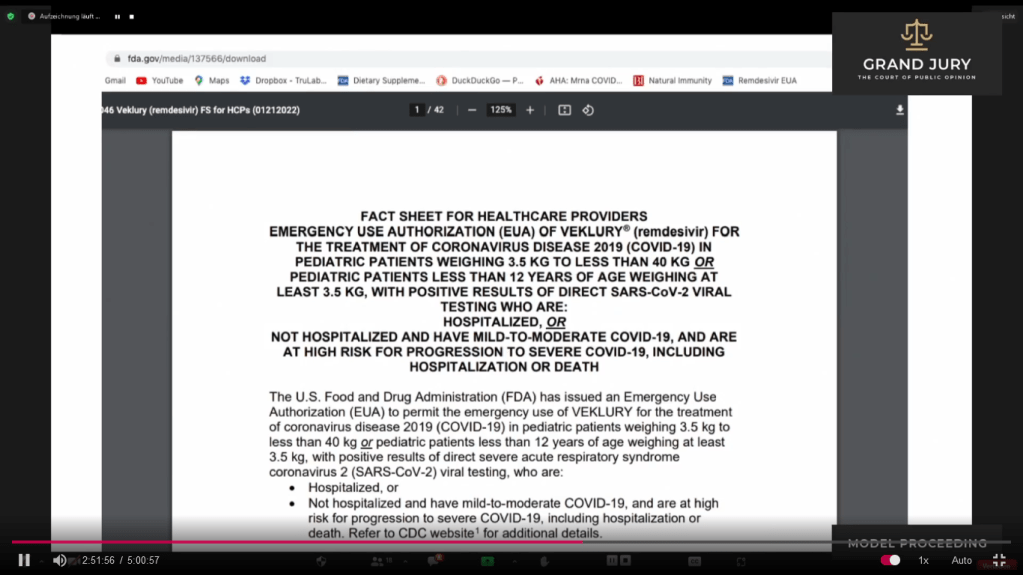
02:51:47 – 02:53:00 Ardis points out that 3 days before FDA came out with the following: “Coronavirus (Covid-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat Covid-19 Due to Omicron Variant” January 24, 2022, (Comment: See slides 14, 14A, 14B ) FDA came out with an Emergency Use Authorization (EUA) of Veklury (remdesivir) for the Treatment of Coronavirus Disease 2019 (Covid-19) in Pediatric Patients….. (Comment: See Slide 15)
(Comment: So the EUA was issued on January 21, 2022, however when you go to FDA to find document, it is a download. The actual document does not show a date and on my particular download platform no date was shown either. However the Gilead Sciences website has a press release regarding the aforementioned EUA which shows the date January 21, 2022. See link for press release: https://www.gilead.com/news-and-press/press-room/press-releases/2022/1/fda-approves-veklury-remdesivir-for-the-treatment-of-nonhospitalized-patients-at-high-risk-for-covid19-disease-progression)
Ardis discusses the aforementioned Emergency Use Authorization Act, what it entails.
02:53:00 – 02:53:25 Slide 15A In regards to the treatment of pediatric patients with Veklury (Comment: Previously known as Remdesivir) Ardis discusses the bullet points at the top of the page, especially the third bullet point “Information on available alternative treatments and the risks and benefits of those alternatives” (Comment: Have to be provided to parent/guardian) (Comment: See arrow on Slide 15A)
Slide 15A FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….”

02:53:31 Slide 15B FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….” (Comment: Slide 15B is shown but not discussed at this time in the presentation).
02:53:35 -02:53:55 Slide 15C FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….”
Ardis points out what is written in regards to approved available alternatives for the treatment of Covid 19 for Pediatric patients, within the prescribed guidelines set out in the title of the fact sheet, that there are no other treatments outside of Veklury (Comment: Previously known as Remdesivir). (Comment: See Slide 15C with arrow, page 10)
Slide 15C FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….” (See arrow)

02:53:55 – 02:54:07 Ardis then points out that again points out on Slide 15A, third bullet point, that information be provided for alternative treatment. (Comment: For convenience, Slide 15A is again shown below)
Slide 15A FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….”

02:54:08-02:54:39 Slide 15D Ardis discusses that this slide points out what the medical community has to do in regards to reporting adverse effects from Remdesivir (Comment: Now known as Veklury. See arrow pointing to #7).
Slide 15D FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….”

02:54:38 – 02:55:07 Slide 15E FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….”
Ardis points out under #8 (Comment: See arrow and asterisks below #8), that the serious adverse events are defined.
Slide 15E FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….” (Comment: See arrow pointing to #8)

02:55:12 – 02:55:30 Slide 15F Ardis points out the following at bottom of slide 15F “FDA has issued the EVA, requested by Gilead Sciences Inc and based on their submitted data. As a healthcare provider, you must comply with the mandatory requirements of the EUA“
Slide 15F FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….”
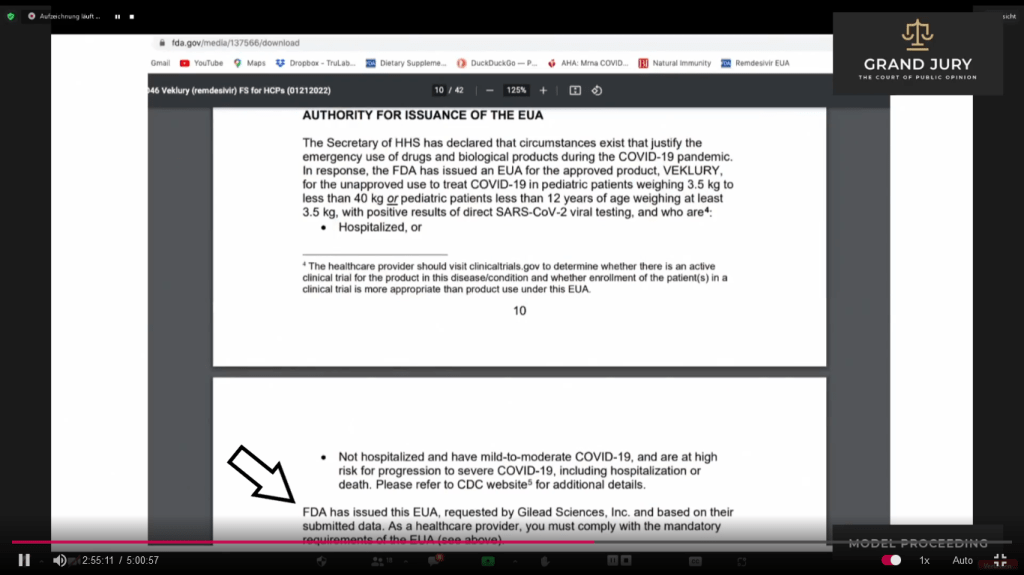
02:55:30 – 02:55:48 Ardis then continues to further discusses the aforementioned information on Slide 15F (Comment: See arrow)
ScreenShot 15F.1 FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….” (Comment: This is not part of the presentation but I’ll type up the first paragraph which is shown with arrow in ScreenShot 15F.1 because I am a little confused “AUTHORITY FOR ISSUANCE OF THE EUA”
“The Secretary of HHS has declared that circumstances exist that justify the emergency use of drugs and biological products during the COVID-19 pandemic. In response, the FDA has issued an EUA for the approved product, VEKLURY, for the unapproved use to treat COVID-19 in pediatric patients weighing 3.5 kg to less than 40 kg or pediatric patients less than 12 years of age weighing at least 3.5 kg with positive results of direct SARS-Cov-2 viral testing, and are4: …… ” [The 4 refers to the small type at the bottom of page 10]. So my question is this: In the second sentence it says “In response, the FDA has issued an EUA for the approved product, VEKLURY, for the unapproved use to treat COVID-19 in pediatric patients.……” I am a little confused to the section that I have highlighted. What does this really mean? Does this refer to what is being pointed out in Slide 15G “11.3 Pediatric Use” by Ardis?)

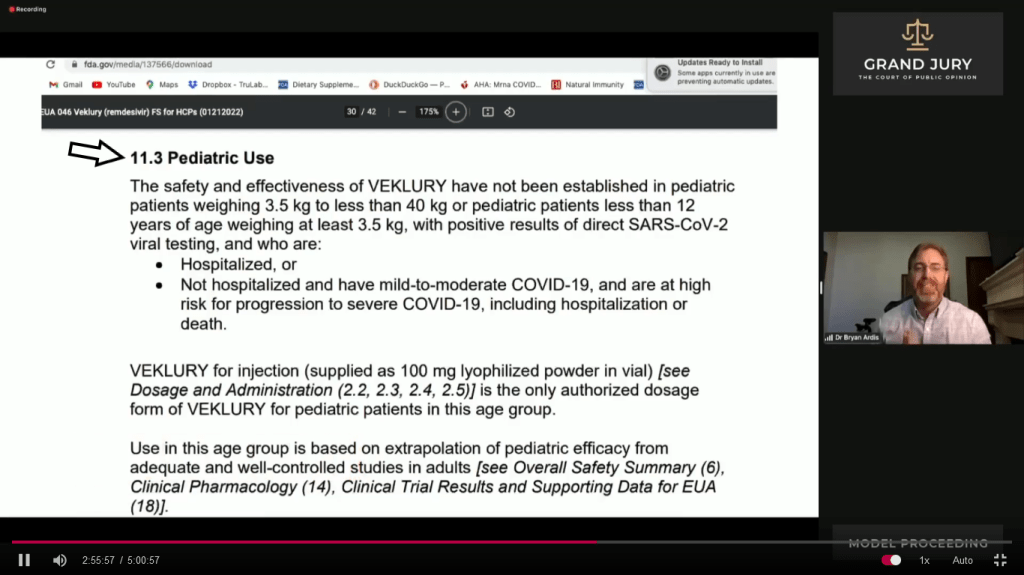
02:55:48 – 02:56:25 Slide 15G “11.3 Pediatric Use”. Ardis discusses what is written under 11.3 (Comment: See arrow) in part it says “The safety and effectiveness of VEKLURY have not been established in pediatric patients…..”
Slide 15G FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….” 11.3 Pediatric Use (Comment: See arrow)
02:56:27 Ardis then refers back to the chart “All mortality results (all stages) (Comment: For convenience see Slide 15H). Ardis notes emphasizing the lack of effectiveness of Remdesivir (Comment: Now called VEKLURY) in treating Covid-19 patients. See highlighted section at bottom of chart).
Slide 15H CHART “All Mortality Results (All stages)” February 13, 2022.

02:56:36 – 02:56:46 Ardis then makes concluding statements to his presentation.
Ardis then takes questions.
02:57:23 Ardis refers back to Slide 15G answering questions.
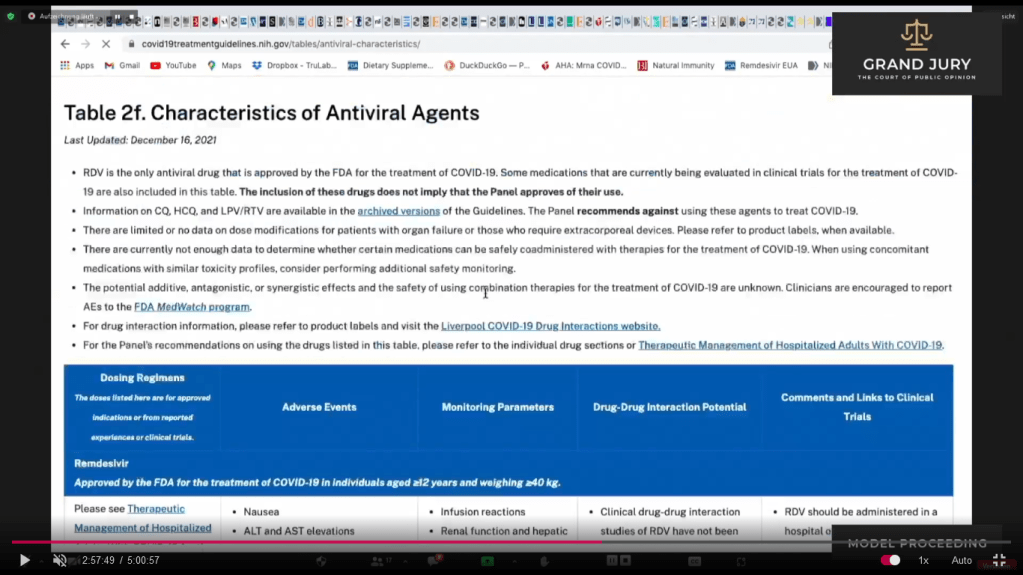
02:57:47 Ardis then refers back to NIH “Table 2f Covid-19 Treatment Guidelines Characteristics of Antiviral Agents December 16, 2021 (Comment: When I was putting in the link for document I realized that it has now been updated as of February 24, 2022. Remember Ardis was discussing the December 16, 2021 Table 2f as part of his presentation made on February 13, 2022. Table 2f was updated 11 days after Ardis’s presentation).
Slides 16 – 16I NIH COVID-19 Treatment Guidelines Table 2f. “Characteristics of Antiviral Agents”. December 16, 2021
(Comment: This table has now been updated as of February 24, 2022, 11 days after Ardis’s presentation, see attached link: https://www.covid19treatmentguidelines.nih.gov/tables/antiviral-characteristics/
02:57:47 Slide 16 NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021
Slide 16A NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. Remdesivir (Comment: Now known as VEKLURY)
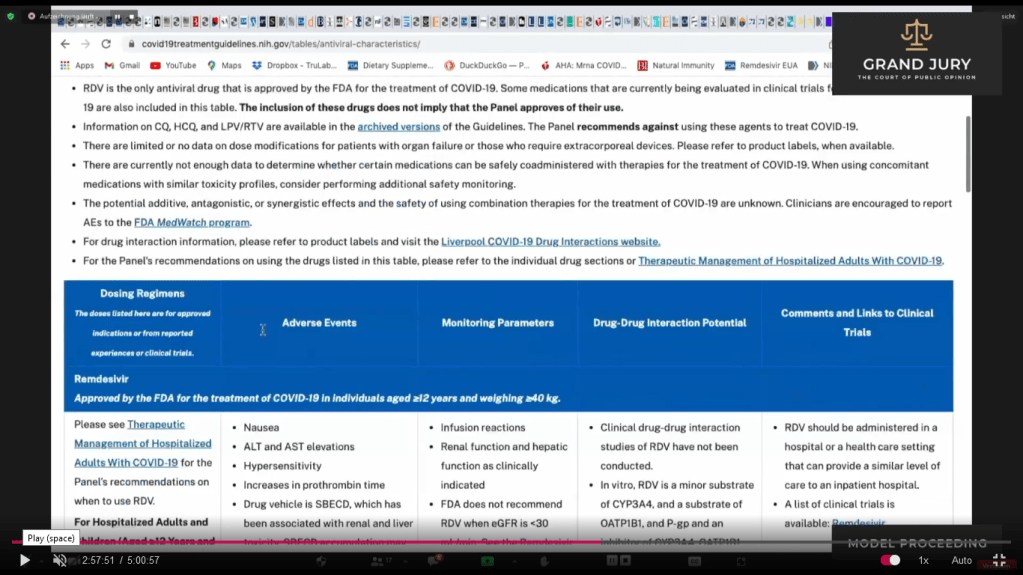
02:57:53 Slide 16B NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. Remdesivir (Comment: Now known as VEKLURY)
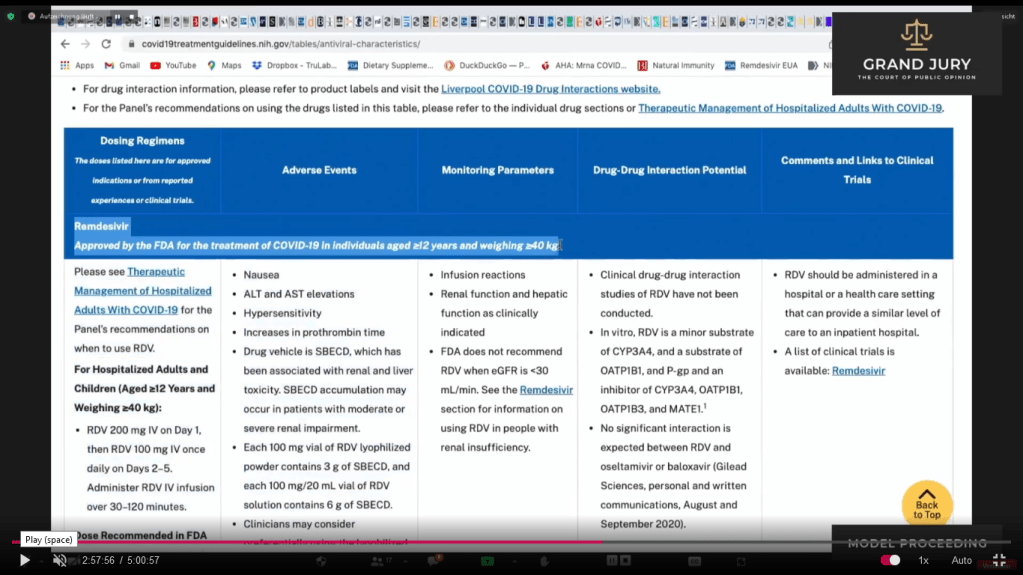
Slide 16C NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. Remdesivir (Comment: Now known as VEKLURY)

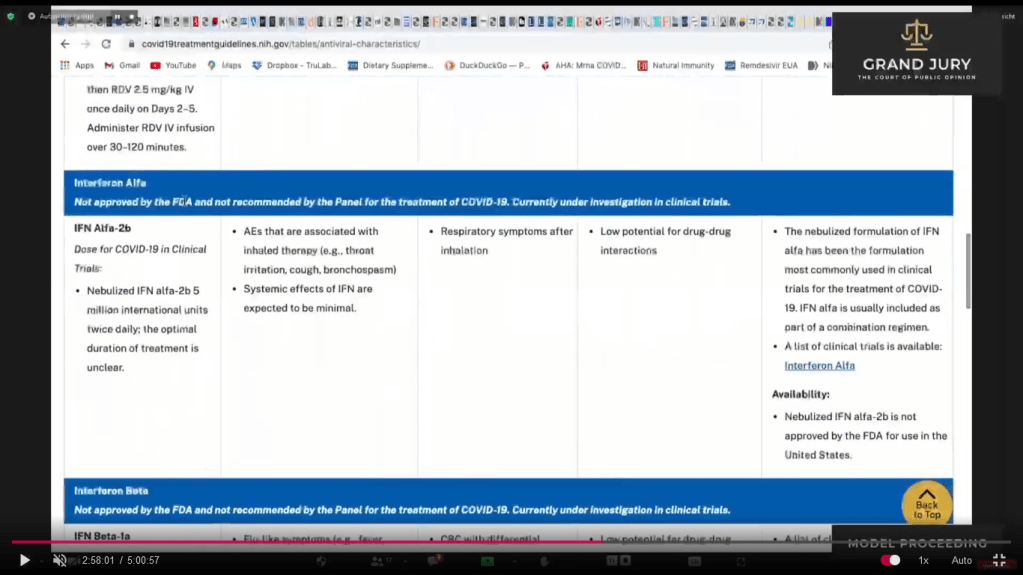
02:58:01 Slide 16D NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021 Interferon Alfa
02:58:06 Slide 16E NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. Interferon Beta
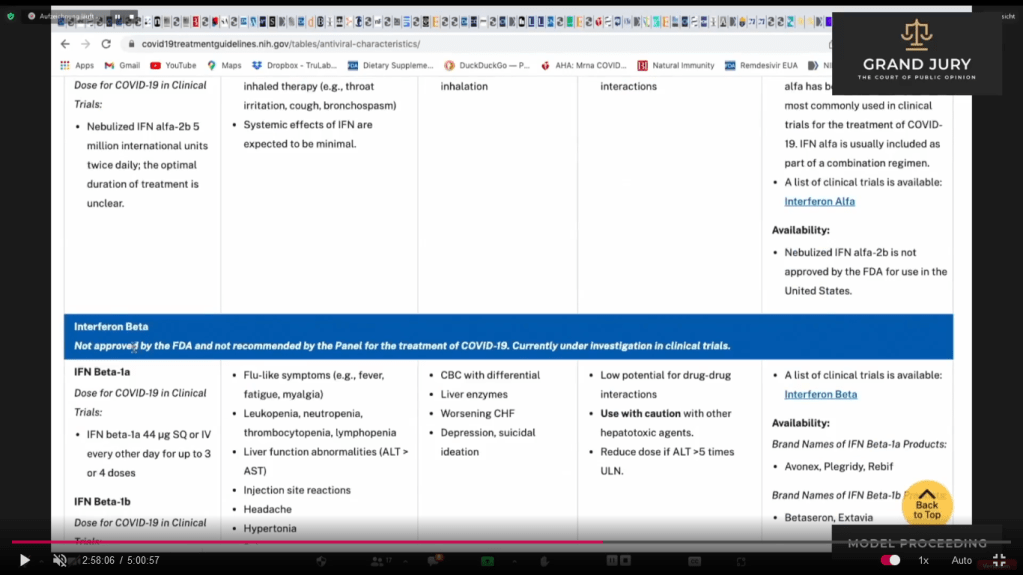
02:58:09 -02:58:10 Slide 16F NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. Interferon Beta
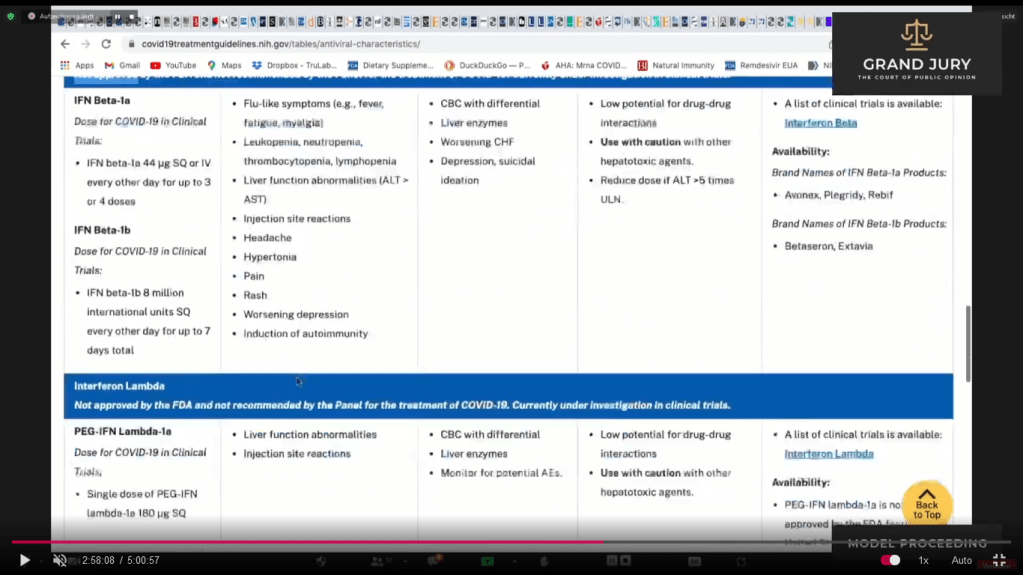
02:58:10 – 02:58:13 Slide 16G NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. PEG-IFN Lambda-1a and Ivermectin
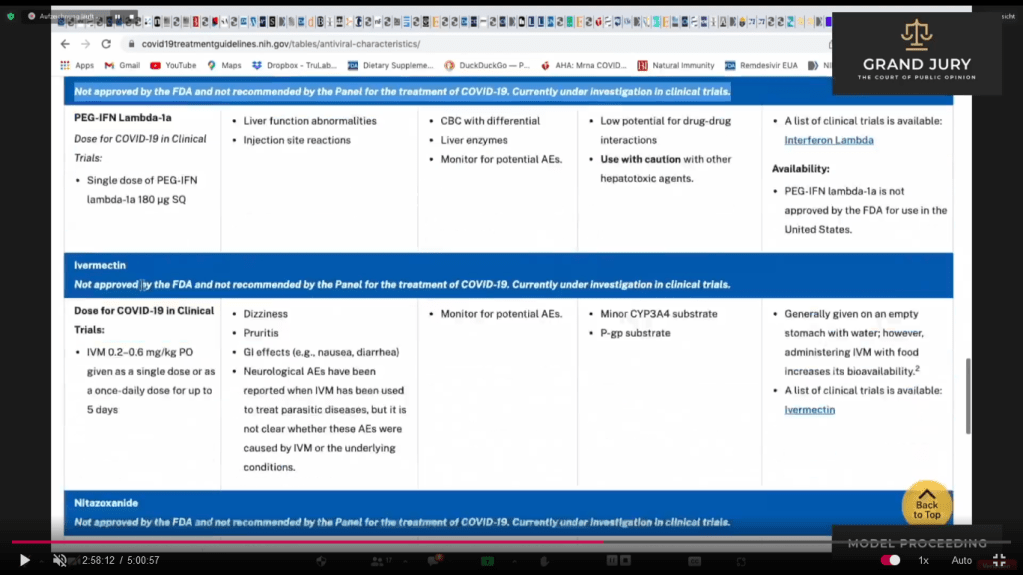
02:58:14 – 02:58:18 Slide 16H NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. Ivermectin and Nitazoxanide
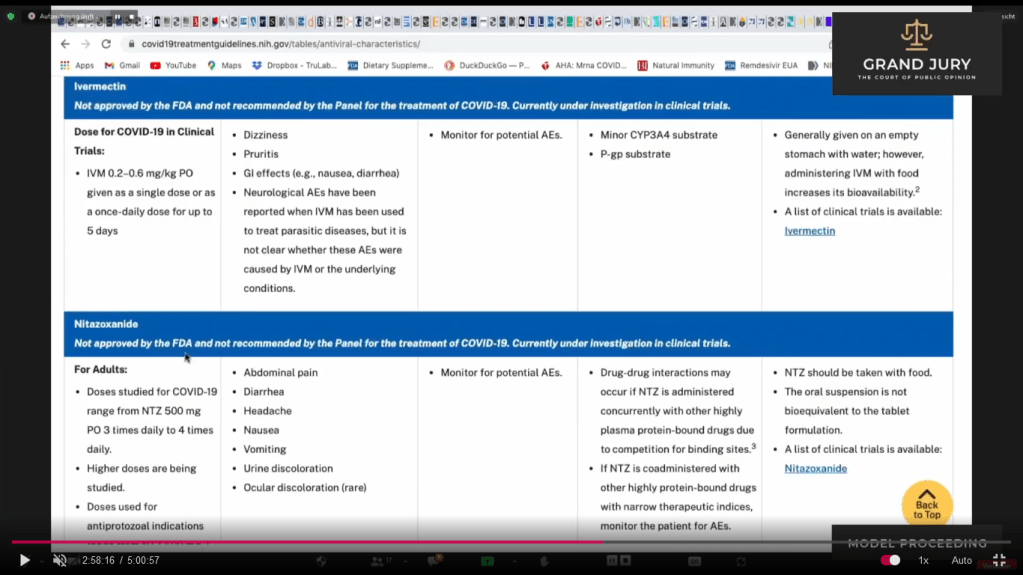
02:58:33 Slide 16I NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. Remdesivir. Ardis discusses Remdesivir’s adverse events in regards to highlighted text.
02:58:33 Slide 16I NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. Remdesivir. (Comment: Highlighted text)
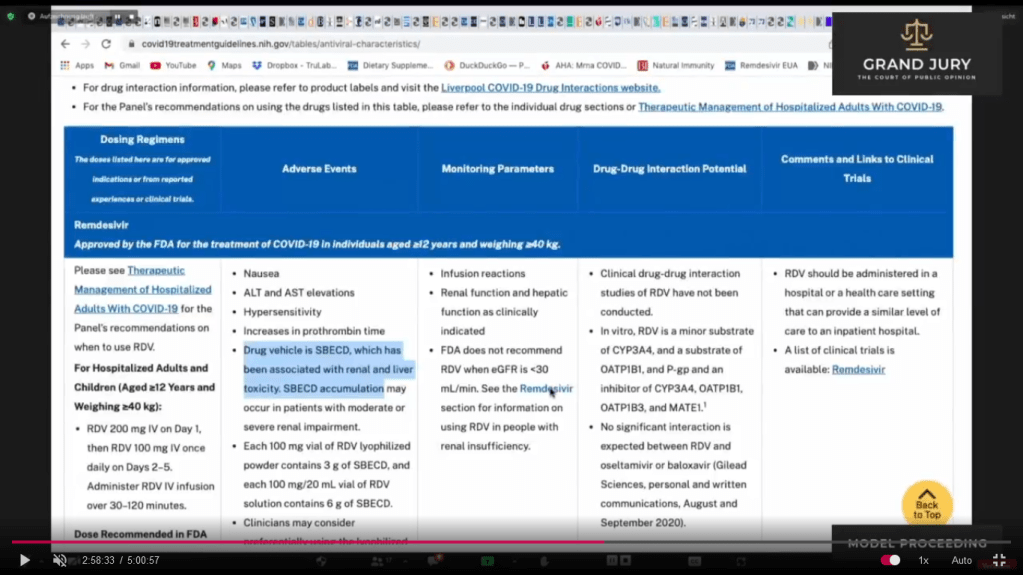
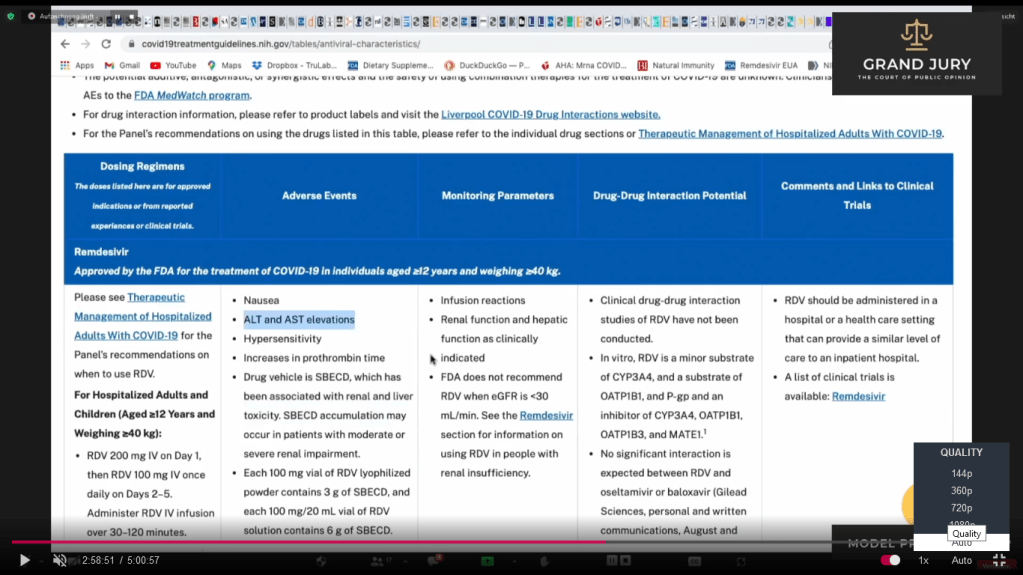
02:58:51 – 02:59:23 Slide 16J NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. Remdesivir. (Comment: Highlighted text). Ardis points out that in the French study regarding the treatment of Covid-19 with Remdesivir (Comment: Refer back to 02:32:18 Slide 3 Open Access, Pub June 30, 2020; Case report: September 01, 2020 : Case report study of the first five Covid-19 patients treated with Remdesivir in France.) Ardis noted that Remdesivir was pulled from the French study when liver enzymes went up between 3 to 5 times its range while in the U.S. Remdesivir is being used with the liver enzyme level is up to 10 times the normal range (Comment: See Slide 17)
Slide 16J NIH Table 2f. Characteristics of Antiviral Agents December 16, 2021. Remdesivir. (Comment: Highlighted text)
02:59:25 – 3:00:35 Slide 17 FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….” Last paragraph on page 7 first bullet point Ardis points out that FDA notes “Consider discontinuing VEKLURY (Comment: Previously known as Remdesivir) if ALT levels increase to greater than 10 times the upper limit of normal” (Comment: See arrow)
Slide 17 FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONAVIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….” Page 7
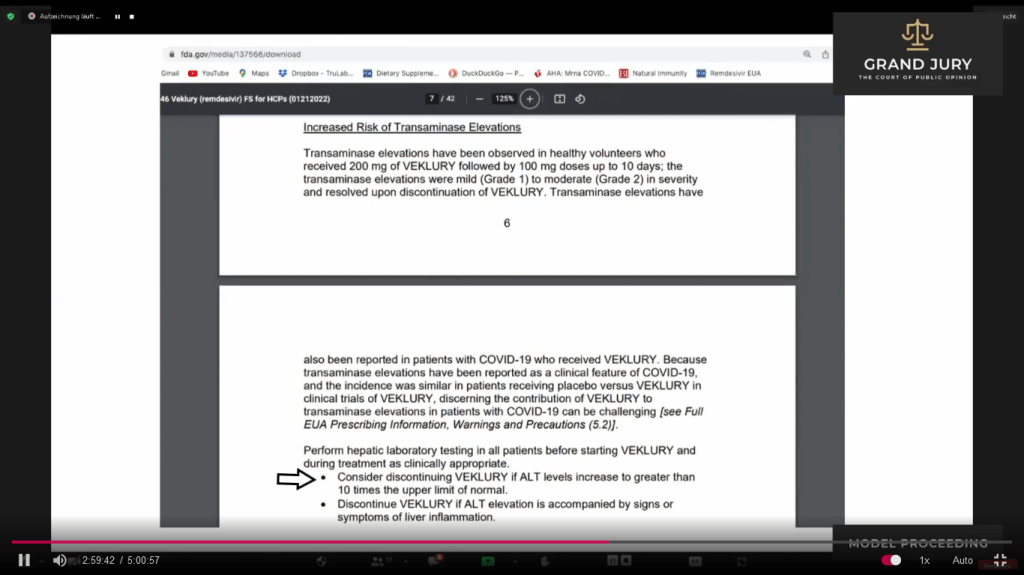
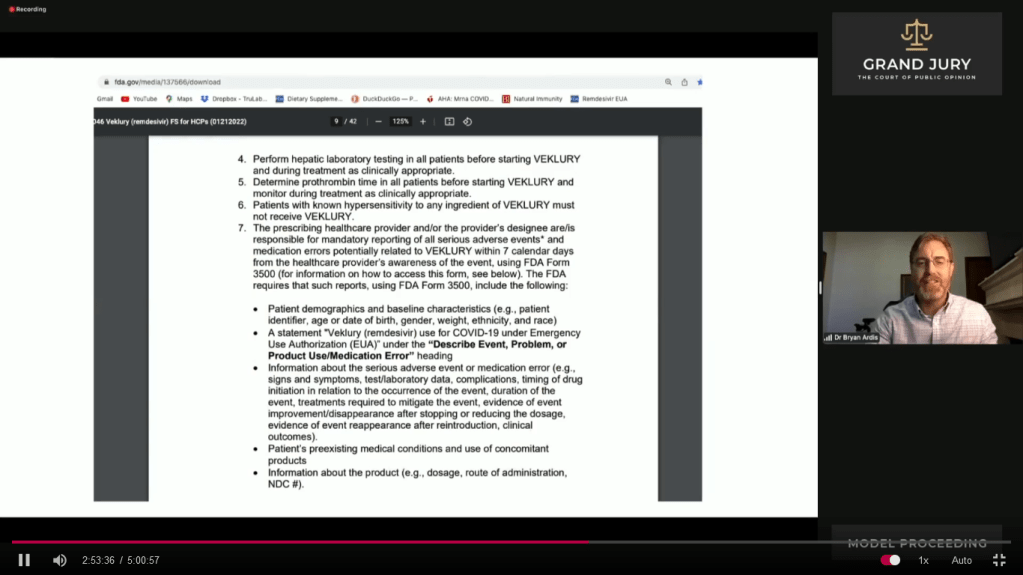
03:01:27 – 3:02:27 Slide 17A FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONA VIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….” Page 9, Number 4 (Comment: See arrow).
Ardis points out the testing requirements for patients that need to be accomplished before administering VEKLURY (Comment: Previously known as Remdesivir) and the testing requirements during the administering of VEKLURY. On #4 (Comment: See arrow) Ardis notes that requirement for performing heptic lab testing. (Comment: Heptic pertains to the liver). Ardis further explains information. Also discusses #5
Slide 17A FDA “FACT SHEET FOR HEALTHCARE PROVIDERS EMERGENCY USE AUTHORIZATION (EUA) OF VEKLURY (REMDESIVIR) FOR THE TREATMENT OF CORONA VIRUS DISEASE 2019 (COVID-19) IN PEDIATRIC PATIENTS…….” Page 9, Number 4 (Comment: See arrow).

03:03:04 – 03:04:13 Ardis continues to summarize his findings and gives hypothesis. Ardis noted the following: “Remdesivir is targeting and killing the elderly. It is complicating issues the liver failure, kidney failure and heart failure, but there are sedation drugs being used around the world, like midazolam, morphine. Currently in America and Canada they are using drugs like this to intubate these people who are being forced into having multiple organ failure, requiring them to go onto a vent (Comment: ventilator) because they can’t breath, and these drugs are midazolam, morphine, lorazapam, precedex, these drugs suppress the nervous system’s ability to control your ability to breath. They are respiratory suppressive. They are also known to paralyze the central nervous system and stop the heart from beating. It is my opinion that they are using these as euthanizing agents and protocols to speed up the actual death processes in hospitals. And they are targeting the elderly around the world, they did from the beginning. And we absolutely see that they are now targeting the young and now all people. And if of interest, if you don’t mind, can I show you one more thing, they are targeting the hearts of all these people with these drugs with sedation and Remdesivir as proven in those studies.”
03:04:14 Slide 18 Publication called “Circulation” dated 08 November 2021. (Comment: Link to publication and article: https://www.ahajournals.org/doi/10.1161/circ.144.suppl_1.10712. This appears to be the same article however the title has been changed).
03:04:23 Ardis states: “I believe there is an asserted attack on heart muscle using Remdesivir, using spike proteins, and using the vaccine. You have to just see this, it goes right along the lines of Remdesivir’s proven to do.”
03:04:36 – 03:04:58 Ardes then discusses what is in the publication “Circulation”, published November 08, 2021.
Slide 18 “Circulation” dated 08 November 2021

03:04:58 – 03:06:38 Slide 18 A “Circulation” dated 08 November 2021 (highlighted text). Ardis discusses information highlighted at end of abstract.
Slide 18 A “Circulation” dated 08 November 2021 (highlighted text)

03:06:42 – 03:07:04 There then follows a discussion around Fauci and possible conflicts of interest involving the drugs, such as financial, that were going to be used for treatment of Covid-19.
03:07:05 – 03:07:30 Ardes discusses a book that is a good reference regarding this matter by Dr. Peter Breggin that had a subtitle of “We are the prey” (Comment: Found referenced book on the internet – “COVID-19 and the Global Preditors: We are the Prey” by Peter R. Breggin MD and Ginger Ross Breggin, Published by Lake Edge Press, Publication Date: September 30, 2021, 690 pages. See link for more details: https://www.wearetheprey.com/)
03:07:31 -03:08:57 Ardes noted that people need to look into connections between Gilead Sciences, Genome Tech, Roche and PacBio. (Comment: Found the websites for all of the aforementioned companies mentioned by Ardis along with a link to their websites: Gilead Sciences: https://www.gilead.com/; Genome Tech: https://genome-tech.org/; Roche: https://www.roche.com/ and PacBio: https://www.pacb.com/).
03:08:11 – 03:09:15 Ardis notes the following: “The selection still, the support still, of using Remdesivir, in my mind, is still to target the hearts, livers, kidneys of individuals. They know its toxic. The reason why I believe they are right now putting Remdesivir as of January 21 with the EUA as the only drug authorized to be pumped into the veins of all children, and there is no other alternatives, as they need babies to start dying because February 15 was the FDA meeting that was supposed to be happening to vote to give these vaccines to newborns, to five year olds, the Pfizer shots. So I believe they are actually setting this up, one step after another, in lockstep, to create more carnage, more death, more trauma, blaming it on a virus, like this Omicron variant, that is almost the common cold, and I would argue that it actually is the common cold, personally. They want actually to project that this is a deadly even in pediatric demographics, so they can sell parents, grandparents, that they need to get the vaccines as well. That is the only reason why they would do it. I can’t find any other reason why you would do it.”
03:09:15 Ardis again refers back to the chart showing the mortality rates from drugs being used to treat Covid-19 throughout the world. Ardis points out that Remdesivir still has only a 19% success rate in treating Covid-19 patents. See Slide 15H
Slide 15H CHART “All Mortality Results (All stages)” February 13, 2022.
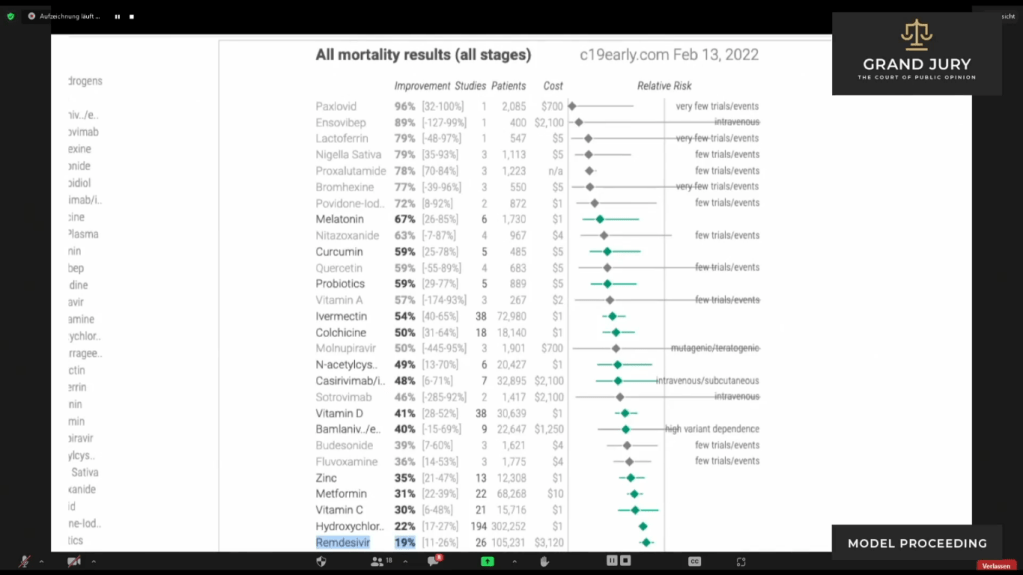
03:09:30 – 03:09:40 Ardis poses the following question, “Why are we still promoting this one and only drug? Its now actually authorized to be used in all nursing homes in America, by the way, outside of hospitals, as of the January 24 (2022) FDA update.”
03:09:45 Ardis then continues: ” So, just like midazolam, morphine being used in the UK to kill innocent elderly people there, call it Covid-19, they are now setting the stage to do it here with a drug called Remdesivir and with the published documentation I gave you there is no reason on the planet I would consider it safe to be given to the elderly, when in fact, right now, February 13, 2022, there’s less than one percent of the entire world that has gotten this virus, been infected with it, who has died, less than 1%.”
03:10:11 – 03:10:57 Ardis then notes the following: “In the state of New York we pulled CMS data with Attorney Thomas Renz and his CMS whistleblowers. The entire state of New York, alone, every Medicare patient who have received five days of Remdesivir treatment for Covid-19, in the state of New York, the entire mortality rate is 26.9%, of all of them died. When I say they are targeting the elderly, they know these drugs can be toxic to them. So, anyway, these numbers are disturbing. Why would you use a drug that is fatal, at this extent, continue on two years later, unless there is an attempt and a plan of genocide. To then sell massive amounts of public around the world that you need our vaccines which are obviously failing.”
03:11:05 More questions are asked.
03:11:33 – 03:12:17 Dr. Reiner Fuellmich, an international trial lawyer, posits the following: “Is it a fair conclusion after looking at everything, all of the evidence that we have heard tonight including your own evidence, is it a fair conclusion to say that: 1. We only have an illusion of a pandemic that was created by a faulty PCR test; 2. They are using this illusion in order to use drugs that kill people which is then; 3. The reason for making people believe that this is caused by the virus, the illusion, and then 4. Use vaccines that are just as deadly as Remdesivir and the other drugs?”
03:12:19 – 03:13:52 Ardus responds: “The illusions (Comment: unintelligible – frame froze) absolutely is the illusions. However, the corruption cannot be understated. Dr. Pierre Kory, he continues to say that this is a non-stop obvious corrupt attempt to create death and harm. The PCR tests do not determine if you have SARS-CoV-2 or Covid-19 or any respiratory virus for that matter, it is a faulty test, faulty cycle set up for it, to create and exaggerate cases of Covid-19. In March of 2020 our CDC published documents to hospitals around the country. Thomas Renz filed a lawsuit – they actually said in March of 2020, all hospitals, if a patient comes in, you test them positive for influenza A or B, but they test negative for pneumonia, and negative for PRC test of Covid-19, this is in their own documents, if you look at the intake form and look at their home address, if its in a city where in the media you have heard there were positive cases of Covid-19, you can call this case a positive Covid-19 case, document it as such, and we’ll give you a 20% additional bonus for that diagnosis, instead of the actually tested confirmed flu diagnosis. And then they did the same thing for pneumonia. So beginning March of 2020, they started incentivizing hospitals and clinicians to give diagnosis of Covid-19 that were false, even if they didn’t test positive PRC, and they incentivized them all to do it. So yes, it is an illusion, orchestrated by our federal health agencies, to exaggerate the cases of Covid, to exaggerate the causes of deaths from Covid, and they’re calling them complications of Covid. And I would definitely call on as many plaintiffs as possible, of loved ones who have died in hospitals around the world, who were treated with Remdesivir. I have actually encouraged, asked for, and have had received thousands of these around the world. I asked them to look at their certificates, their death certificates, and you’ll see that they died of complications of Covid-19, diagnose secondly from acute kidney failure, and they died from complications of secondary Covid pneumonia. It is not secondary Covid pneumonia, when you shut down the kidneys and you put a IV bag, flooding water into the veins of someone, and they can’t excrete the water from their kidneys you’ve shut down, you flood their abdomen with water, it then floods inside the heart, and it infiltrates your lungs and you drown them to death. It is called pulmonary edema. So I call on everybody to drive as many of these people to actually give this information. We know that this is what the drugs do. Yes, there is an illusion that they are dying from a virus, they are being poisoned to death with ill advised protocol, absolutely.”
03:15:08 Ardis continues: “They also being asked and incentivized to artificially exaggerate positive cases, and give diagnoses for it. I just showed you that Medicare, in America, is incentivizing hospitals with a 20% bonus and the code, if you’ll just give us a positive Covid-19 diagnosis for all Medicare aged patients.”
03:15:29 Ardis continues: “Really, I would like to ask you, jury, judge: If like I showed you on the NIH chart, that there is only one drug, as of December 16 that is FDA approved for treating Covid-19 hospitalized patients, why is Medicare bribing all of the hospitals to use that one and only drug, if there is only one approved to be used anyway. Why would Medicare have to bribe them with a 20% bonus pay out to use the one and only one. There can only be a nefarious reason behind why they are bribing all hospitals to use that one drug proven to cause acute kidney failure, liver failure, and heart failure. From the beginning we knew that was the case. You’ve all been lied to.
03:16:12- 03:19:44 Discussion continues for a little over 3 minutes and then presentation ends with Ardis.
Published: 04/02/2022 at 11:54PM EST

